Circular Economy and Me – Issue 10

Circularity of carbon: selective electrochemical conversion of carbon dioxide to carbon monoxide by Preetam Sharma
Circular economy operates on the principles of reuse, refurbish and recycle. For humanity to stay in the safe operating limits, these principles need to be implemented in all sectors including carbon. For sustaining the current world, most of the carbon is obtained from the fossil sources which are used in a variety of sectors including energy and chemicals. It is possible to de-carbonise the energy sector by using renewable energy sources, but chemicals and manufacturing sectors cannot be de-carbonised due to the requirement of carbonaceous chemical feedstocks. However, de-fossilisation of the chemical industry is possible by the use of recycled carbon. The capture and utilisation of carbon dioxide (CO2) emissions (CCU) by either direct air capture or capture at a point source can be an important route for lowering CO2 levels in the atmosphere. Electrochemical CO2 reduction, which takes CO2 and water along with renewable electricity as the inputs, has the potential to produce variety of fuels and chemicals feedstock. This can help humanity to stride towards United Nations Sustainable Development Goals and Net Zero 2050 target. The captured CO2 can be used to produce a variety of feedstocks including carbon monoxide (CO). CO can be converted to methanol via industrially established methods or petrochemicals via Fisher-Tropsch method. Electrochemical CO2 reduction have technical challenges in terms of low energy efficiency and low reactor operational stability at high current densities. However, with technological advances in the field, these issues can be resolved and the processes can eventually contribute to net zero.
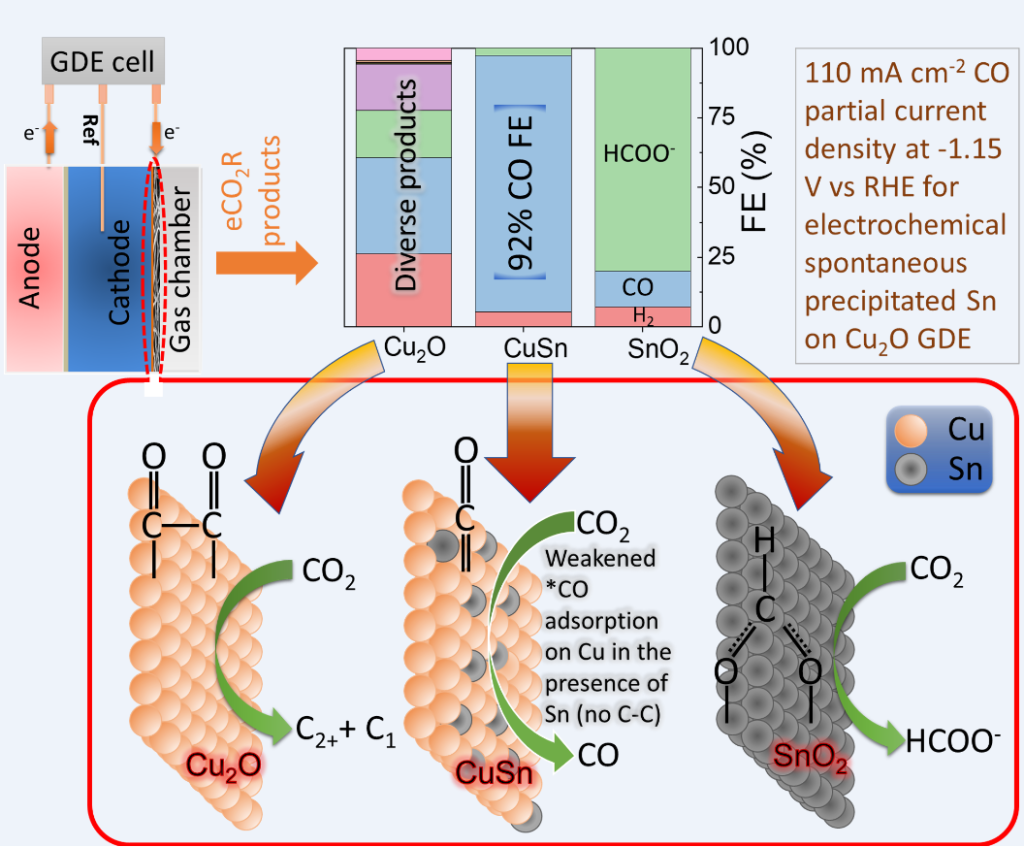
In terms of electrochemical CO2 reduction system, traditional H-cell consisting of anode and cathode chambers separated by an ion exchange membrane suffer from the mass transport limitation of CO2 to the surface of the cathode. Therefore, we have employed a three-chamber gas diffusion electrode (GDE) reactor where the cathodic reactions take place at the gas-liquid interface. In this system, we were able to obtain CO with >90% faradaic efficiency using Cu-In catalysts. Indium is a previous metal and would limit the commercial potential of the system. Via my participation in the circular economy project, we were able to develop earth abundant Cu-Sn electrodes by a facile single-step Sn deposition process. The Cu-Sn electrodes were employed in a three-chamber GDE reactor for determining the CO2 reduction products. For 20 min galvanically deposited Sn, highest CO faradaic efficiency of >92% was obtained at 110 mA cm-2 current density. The encouraging results with CuSn GDEs, provides a possible route to produce CO from CO2 using a low-cost high efficiency catalyst. Currently, we are focusing on improving the GDE stability and scaling-up the GDE reactor from the current 2 x 2 cm2 to 10 x 10 cm2 scale using the low-cost Cu-Sn electrodes.
Apart from the core technology development, integration of these carbon recycling technologies with industrial infrastructure is vital for their implementation. As these technologies once they reach at higher TRLs, will need to integrate with the industrial infrastructure. Therefore, a collaboration with experts from a diverse background is required to integrate these in the future industrial designs and infrastructure. That is why, CircularChem is such an important platform where novel carbon recycling technologies are being developed in collaboration with process engineers, policy expects, industry and business experts.
.
Further reading:
A safe operating space for humanity
United Nations Sustainable Development Goals
Selective conversion of CO2 to CO using earth abundant tin modified copper gas diffusion electrodes