Circular Economy and Me – Issue 9

Converting Methane By-Products to Valuable Chemicals by Ben Lyons.
One of the main principles that a circular economy is based on is the reduction or elimination of waste streams. Despite significant progress in the chemical industry towards waste reduction, there are still unavoidable and undesirable by-products that need to be addressed to achieve a truly circular economy. Carbon dioxide emissions are the most well-known and studied example, but there is an additional area of opportunity that involves another greenhouse gas: methane.
Methane is a common by-product across a wide range of chemical processes, from steam cracking for the manufacture of olefins to the anaerobic digestion of wastewater sludge. Typically, this methane is combusted as a fuel source to either supply process heat directly or to drive a turbine to generate electricity. This usage of methane reduces the need for external energy sources in chemical plants and enhances their economic viability. However, as the industry moves towards a more sustainable and circular future, the combustion of methane is no longer a viable option due to the release of CO2 into the atmosphere and the subsequent loss of valuable carbon from the system. Furthermore, methane emissions, being a potent greenhouse gas, pose a significant environmental threat. Recent studies have demonstrated that flaring, which is a common practice for methane disposal in oil and gas operations, is less effective at reducing emissions than previously assumed (Plant et al., 2022). These issues highlight the critical need to explore alternative pathways for methane utilisation within the chemical sector.
Part of my work as a PhD student in the Centre has been to investigate the potential options for valorising methane by-products. Using tools such as simulation modelling, techno-economic analysis, and environmental assessments, we can evaluate different methane utilisation options to identify the most promising routes. In some recent work, we have been looking at 3 main types of upgrade routes for methane: syngas-based routes, methane plasma pyrolysis, and oxidative coupling of methane (Lyons et al., 2023). In the syngas-based routes, methane is reformed using an oxidant or mixture of oxidants to produce syngas, a mixture of mainly CO and H2. This syngas mixture can then be used to produce methanol, which can further be converted into additional olefins. In plasma pyrolysis, renewable electricity is used to generate a high temperature plasma that decomposes the methane into its elemental components: solid carbon and H2. Finally, oxidative coupling of methane involves reacting methane and oxygen over a metal oxide catalyst at high temperatures to yield ethylene, ethane, and other side products.
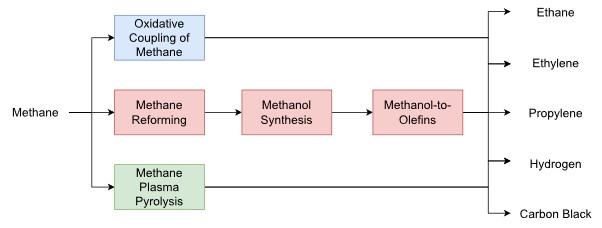
Figure 1: Overview of investigated methane valorisation routes
This work involves developing simulation models for these 3 routes and comparing their economic and environmental performance to a base case scenario (i.e. burning the methane by-product for energy). The preliminary results look promising, with both economic and environmental benefits being realised at certain scales of methane production. This work is still ongoing though and a more thorough analysis is needed to confirm the results. Nevertheless, by identifying the most promising options for methane valorisation, this investigation forms a part of the Centre’s overall objective in promoting a more circular and sustainable UK chemical industry.
References:
Lyons, B. et al. (2023) ‘Methane-to-X: an economic assessment of methane valorisation options to improve carbon circularity’, in. Computer Aided Chemical Engineering, 33 European Symposium on Computer Aided Process Engineering (in review).
Plant, G. et al. (2022) ‘Inefficient and unlit natural gas flares both emit large quantities of methane’, Science, 377(6614), pp. 1566–1571. Available at: https://doi.org/10.1126/science.abq0385.